Pharma Auditor Certification

This course includes a 2 hour written examination.
Pharma auditor certification. Auditing for gmp is specifically designed to address the challenges of gmp auditing for the pharmaceutical industry and present. This auditing for gmp course is specifically designed to address the challenges of gmp auditing for the pharmaceutical industry and present the basic competencies required to effectively perform the auditors assigned responsibilities and contribute to the improvement of auditor performance within a regulated industry. This online course includes a supplemental. Watch a video to learn more about our cqi and irca certified auditor training program.
This course meets the training requirement for irca certification for all grades of pqms auditor. Download the quality auditor certification fact sheet pdf 61 kb. Our pharmaceutical gmp auditorlead auditor course is approved by irca the international register for certificated auditors and meets their current pqms auditor lead auditor training course requirements. Who is it for.
Tutors were very open and extremely knowledgeable. The applicant can join any gmp relevant eca training course or conference within two years. Course materials were extensive. The certified quality auditor analyzes all elements of a quality system and judges its degree of adherence to the criteria of industrial management and quality evaluation and control systems.
Pharmaceutical gmp auditor training. Download the quality auditor certification brochure pdf 328 mb. Many companies now require their auditors to be trained through the nsf certified lead auditor course. The certification is meant to ensure that the successful participant has got the skills needed to assess and report on the conformance and effective implementation of processes and to contribute to the continual improvement of a quality management system based on standard.
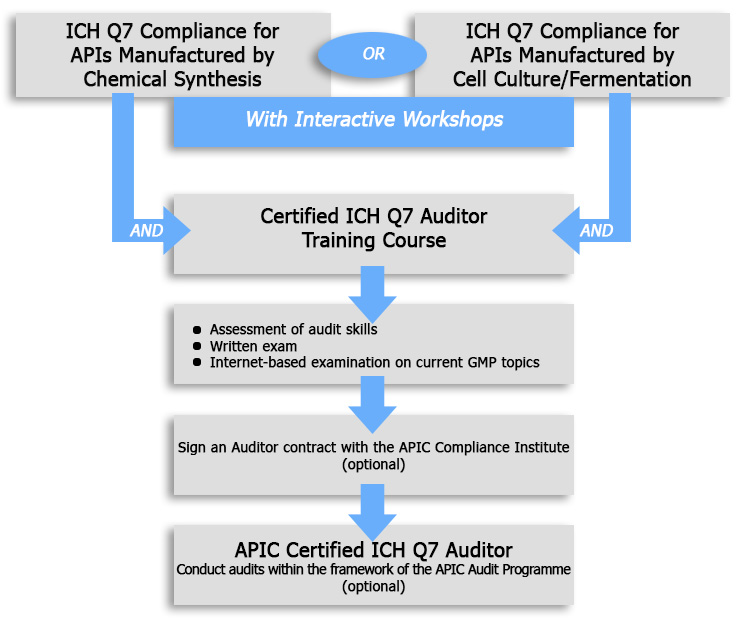
In order to reflect a continuous professional devel opment for gmp auditors this certificate can be ex tended for continuous training. This means you can trust our experts for your training. Auditors must perform their jobs competently to ensure their companys compliance with pharmaceutical usfda gmp regulations and other quality standards like ich q10. This course is for those intending to acquire the knowledge and skills to audit a whole ich q10 based pharmaceutical quality management system either as a third or second party auditor.
Find out more about nsfs pharmaceutical auditor training and audit support services. Here an additional certificate can be obtained eca certified gmp auditor. A minimum of three years of this experience must be in a decision making position.