Gmp Risk Assessment Template
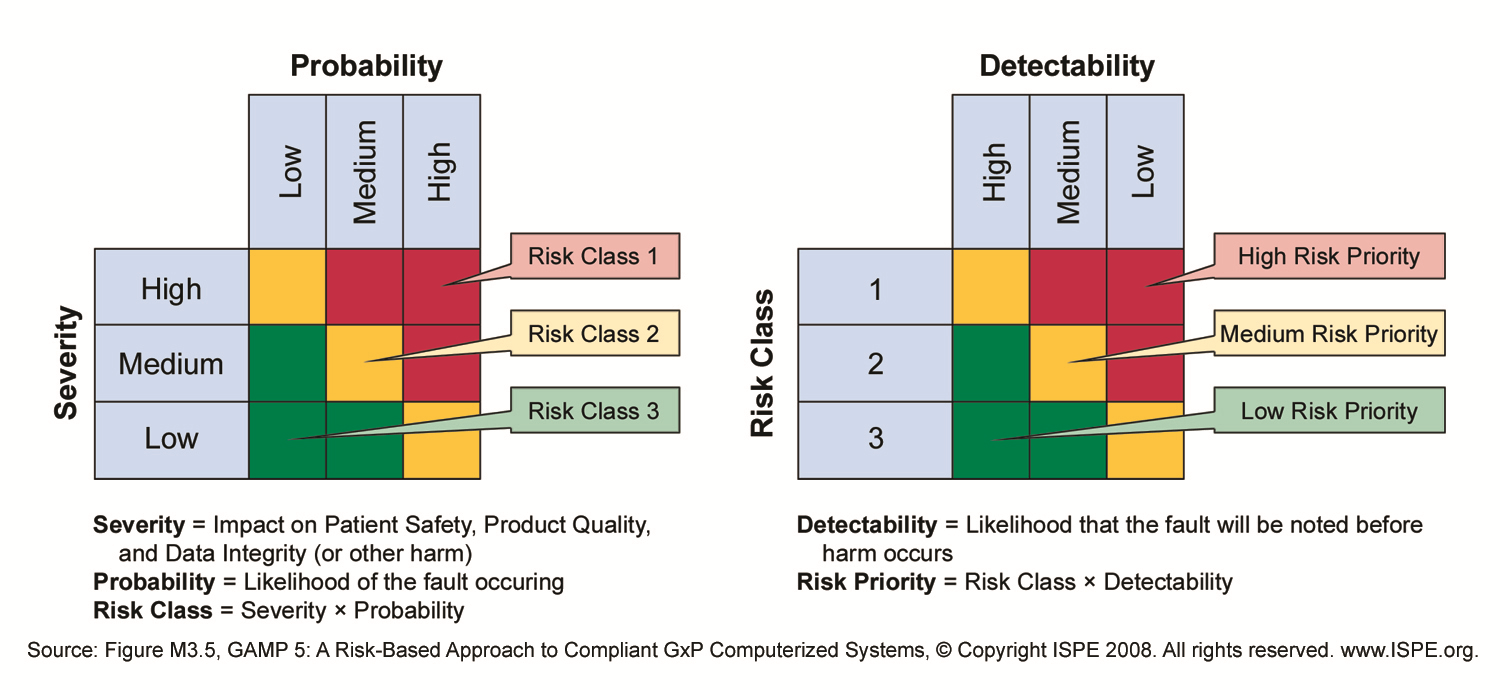
The matrix is based on two variables.
Gmp risk assessment template. Quality risk management page 5 examples of severity level criteria table 2 probability level criteria table 3 and a risk evaluation matrix table 4 are shown below. The risk assessment team determines the severity probability and detectability of the risk. The risk assessment consists in the identification of hazards analysis and evaluation of risks associated with exposure to those hazards risk assessment defines with three fundamental questions. The level of effort formality and documentation of the quality risk management process should be commensurate with the level of risk.
Managers can use this digital template to proactively assess which effective risk control and prerequisite programs are to be used. During risk assessment the probability of occurrence and detectability should be considered and measures to reduce the risk identified. Pharmaceutical standard operating procedure template describes the companys process to be used in conducting a risk assessment. Objective of the risk assessment identification of potential risks related to products preventive corrective actions.
An example for a technical gmp risk assessment is given below. Id contents page no. The risk based qualification assessment describes the qualification steps risk assessment design installation operational and performance qualification etc required to qualify the equipment in order mitigate that risk. This method represents a combination of a quantitative risk assessment based on the risk index method and a qualitative risk ranking method.
Use this template as a guide for the following. For each requirement is defined if the requirement is critical in relation to gmp and a risk scenario is defined. The assessment starts with a list of requirements derived from the user requirements specification urs. 10 responsibilities 20 objective 30 scope 40 risk identification 50 risk assessment and investigations 60 risk mitigation and prevention.
Qarisk assessmentsrisk assessment templates following risk matrix can be used effectively to assess risks derived from a quality incident such as deviation complaint or out of specification investigation. Risk identification assessment mitigation template. The present article therefore describes a new combined quantitative method for assessing excipient risks that has been developed by the authors as one possible risk evaluation method. This taccp risk assessment template can be used to identify critical points in food production that are at risk to threats.
Evaluate the process analyze the hazard.